 R15 MH125306
R15 MH125306 R15 MH125306
R15 MH125306
Yaeger JDW, KT Krupp, BM Jacobs, BO Onserio, BL Meyerink, JT Cain, PJ Ronan, KJ Renner, RJ DiLeone, CH Summers 2022
Orexin 1 receptor antagonism in basolateral amygdala shifts the balance from pro- to anti-stress signaling and behavior.
Biological Psychiatry 91: 841-852
Yaeger JDW, KT Krupp, TR Summers, CH Summers 2022 Contextual generalization of social stress learning is modulated by
orexin receptors in basolateral amygdala. Neuropharmacology 215: 109168:1-12
Carpenter RE, B Sabirzhanov, TR Summers, TG Clark, J Keifer and CH Summers 2023 Anxiolytic reversal of classically conditioned /
chronic stress-induced gene expression and learning in the Stress Alternatives Model. Behavioural Brain Research
114258: 440: 1-10
 R15 MH104485
R15 MH104485
Korzan WJ and CH Summers 2021 Evolution of stress responses refine mechanisms of social rank. Neurobiology of Stress 100328: 1-19 Pang T, JDW Yaeger, CH Summers, and R Mitra 2021 Cardinal role of the environment in stress induced changes across life stages and generations. Neuroscience and Biobehavioral Reviews 124: 137-150 Yaeger JDW, KT Krupp, JJ Gale, CH Summers 2020 Counter-balanced microcircuits for Orx1 and Orx2 regulation of stress reactivity. Medicine in Drug Discovery 100059: 1-20 Summers CH, JDW Yaeger, CD Staton, DH Arendt, TR Summers 2020 Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: potential for therapy. Brain Research 1731: 146085: 1-15 Data from: Staton CD, JDW Yaeger, DD Khalid, F Haroun, BS Fernandez, JS Fernandez, BK Summers, TR Summers, M Sathyanesan, SS Newton, CH Summers 2018 Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology 143: 79-94.
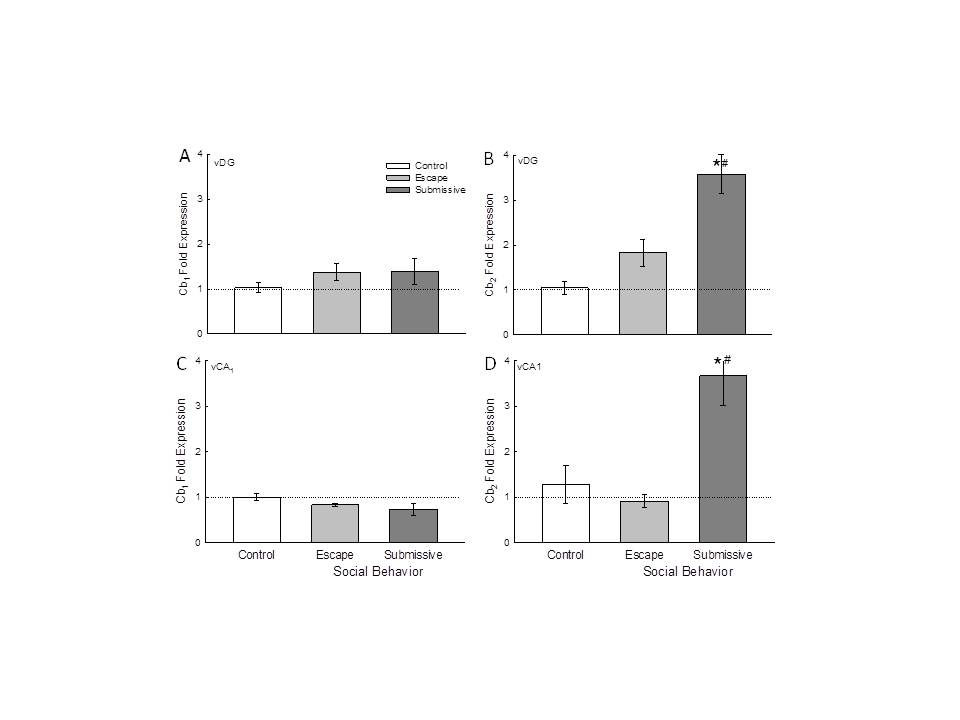 |
Figure 3. - Social stress modifies Cb2 receptor gene expression in the ventral hippocampus. A) Socially aggressive interactions plus fear conditioning in the SAM significantly (* compared with cage controls [white bar], # compared with escape [gray bar]) increased ventral dentate gyrus (vDG) Cb2 receptor mRNA (mean ± SEM) in submissive (dark gray bar) mice. B) In the vCA1 the Cb2 receptor mRNA is significantly (# compared with escape) increased in submissive animals (no change from control animals). |
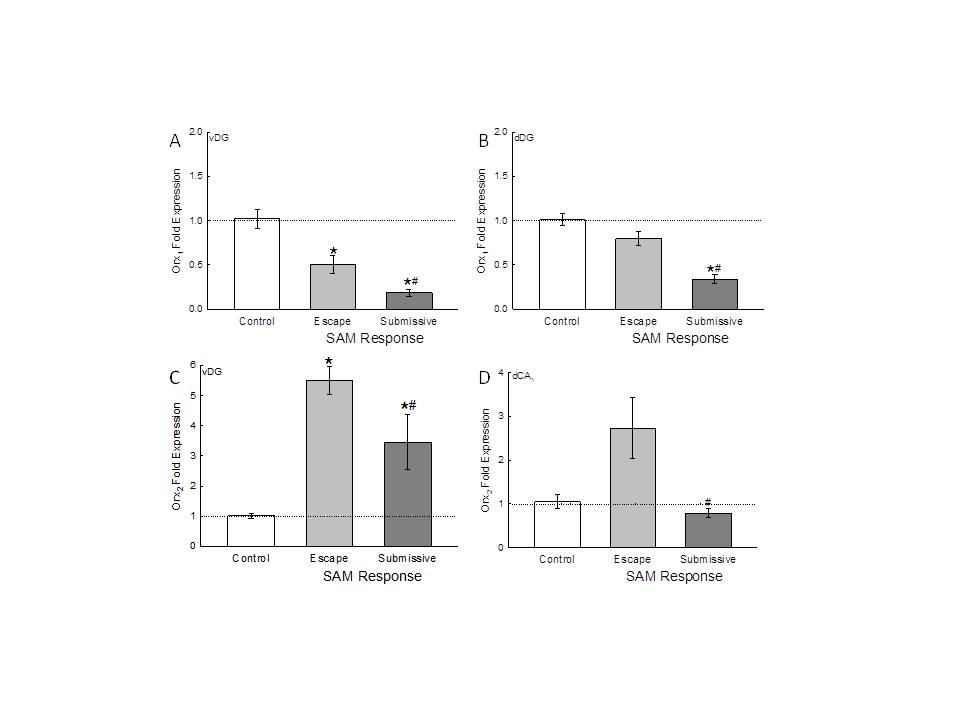 |
Figure 3. - Social stress + fear conditioning (FC) diminishes Orx1, but enhances Orx2, receptor gene expression in the ventral hippocampus. Regardless of subregion, submissive mice always show reduced Orx receptor mRNA compared with escaping mice. A) Interactions in the SAM + FC significantly (*compared with cage controls [white bar]) decreased ventral dentate gyrus (vDG) Orx1 receptor mRNA (mean ± SEM) equally in escaping (gray bar) and submissive (dark gray bar) mice, submissive mice Orx1 receptor mRNA significantly (# compared with escape) decreased from escaping mice. B) Social stress + FC diminishes Orx1 receptor gene expression in the dorsal hippocampus. Interactions in the SAM significantly (*compared with cage controls, # compared with escape) reduced dorsal dentate gyrus (dDG) Orx1 receptor mRNA in submissive mice. C) The mRNA for Orx2 receptor in vDG is significantly (*compared with cage controls) increased in escaping and submissive animals, but with submissive Orx2 mRNA significantly (#) reduced compared with escaping mice. D) Social stress diminishes Orx2 receptor gene expression in the dorsal hippocampus. Interactions in the SAM +FC significantly (# compared with escape) decreased dorsal CA1 Orx2 receptor mRNA in submissive mice. |
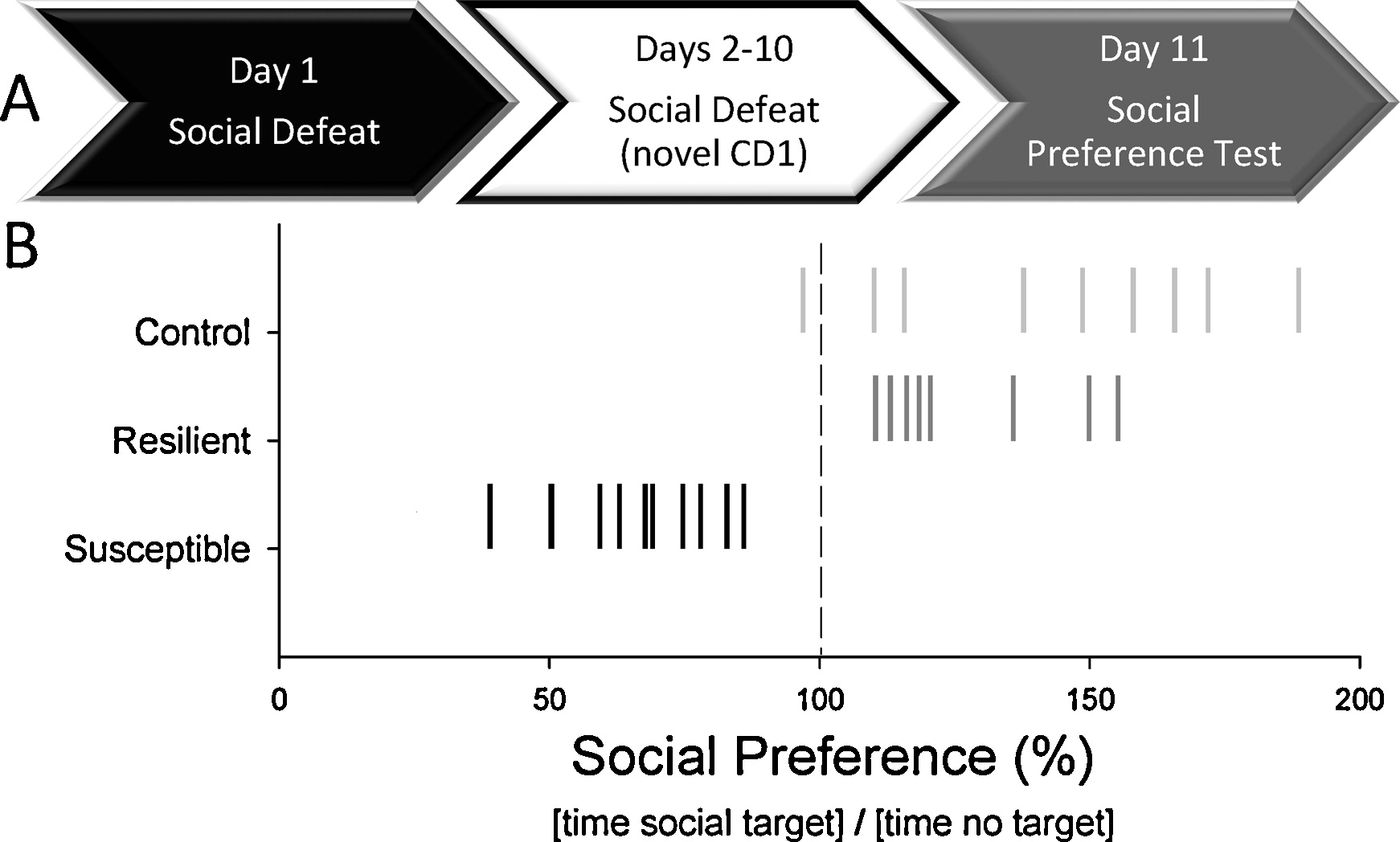 |
Figure 1. - Social Defeat Protocol (A) Sequence and timing of social defeats (by a novel CD1 mouse once/day for 10 days) and following social preference test (day 11). (B) Social preference scores based on the amount of time test mice spent near and away from a social target delineate animals that were resilient and susceptible to social defeat. “Resilient” animals were defeated, but still showed a social preference (>100% time spent with social target/time spent away from the social target × 100) similar to “Controls”. Defeated animals that displayed a social aversion (<100%) relative to controls and were defined as “Susceptible”. |
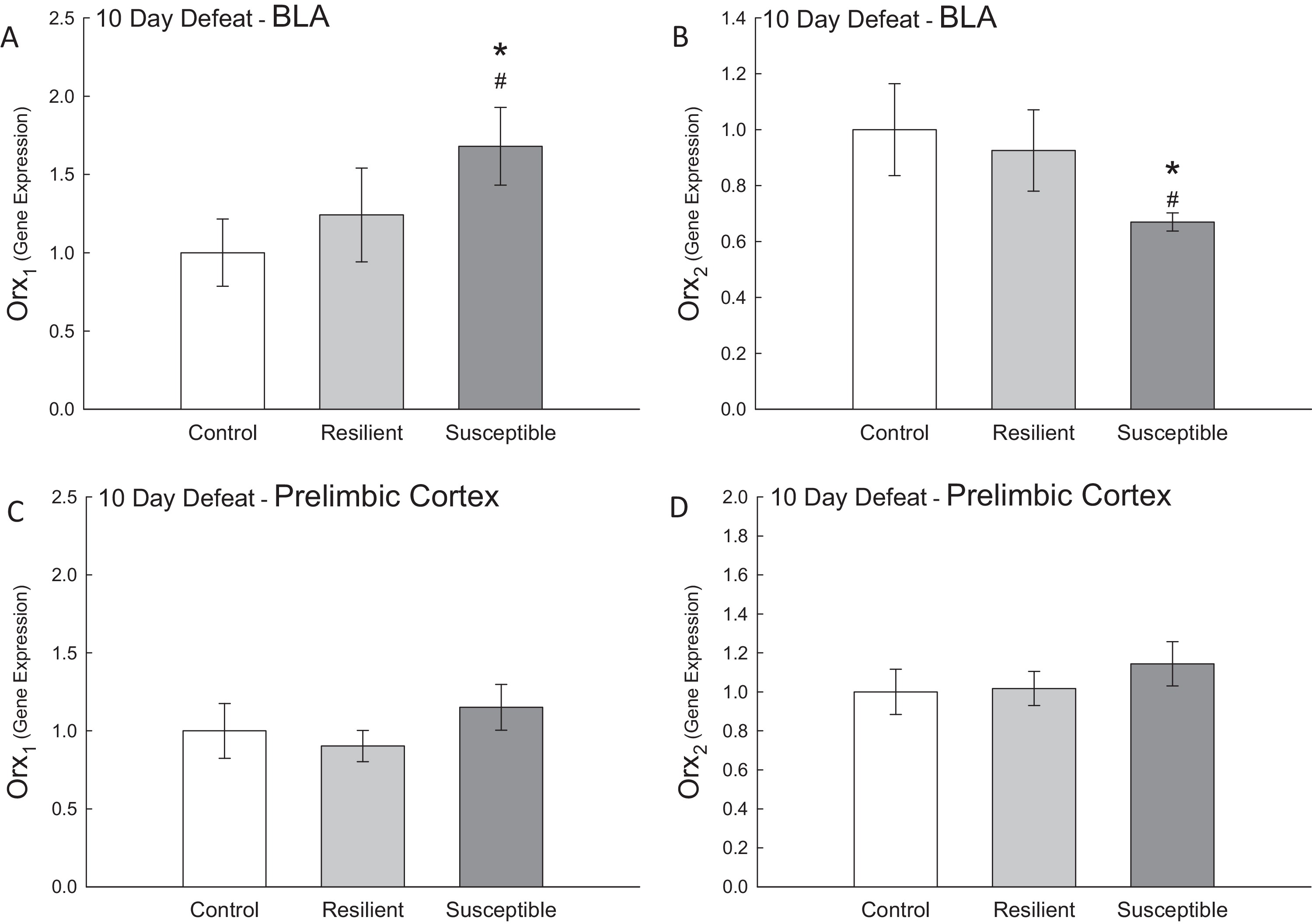 |
Figure 2. Susceptible mice have increased BLA Orx1 but decreased Orx2 receptors In the basolateral amygdala (BLA), (A) susceptible animals had significantly elevated amounts of Orx1 mRNA relative to control (*) and resilient (#) animals. (B) This effect in susceptible animals occurred with a concomitant decrease in BLA Orx2 mRNA. In the prelimbic cortex (PrL), there were no differences between the groups for the (C) Orx1 or (D) Orx2 receptor transcripts. *,#p < 0.05. |
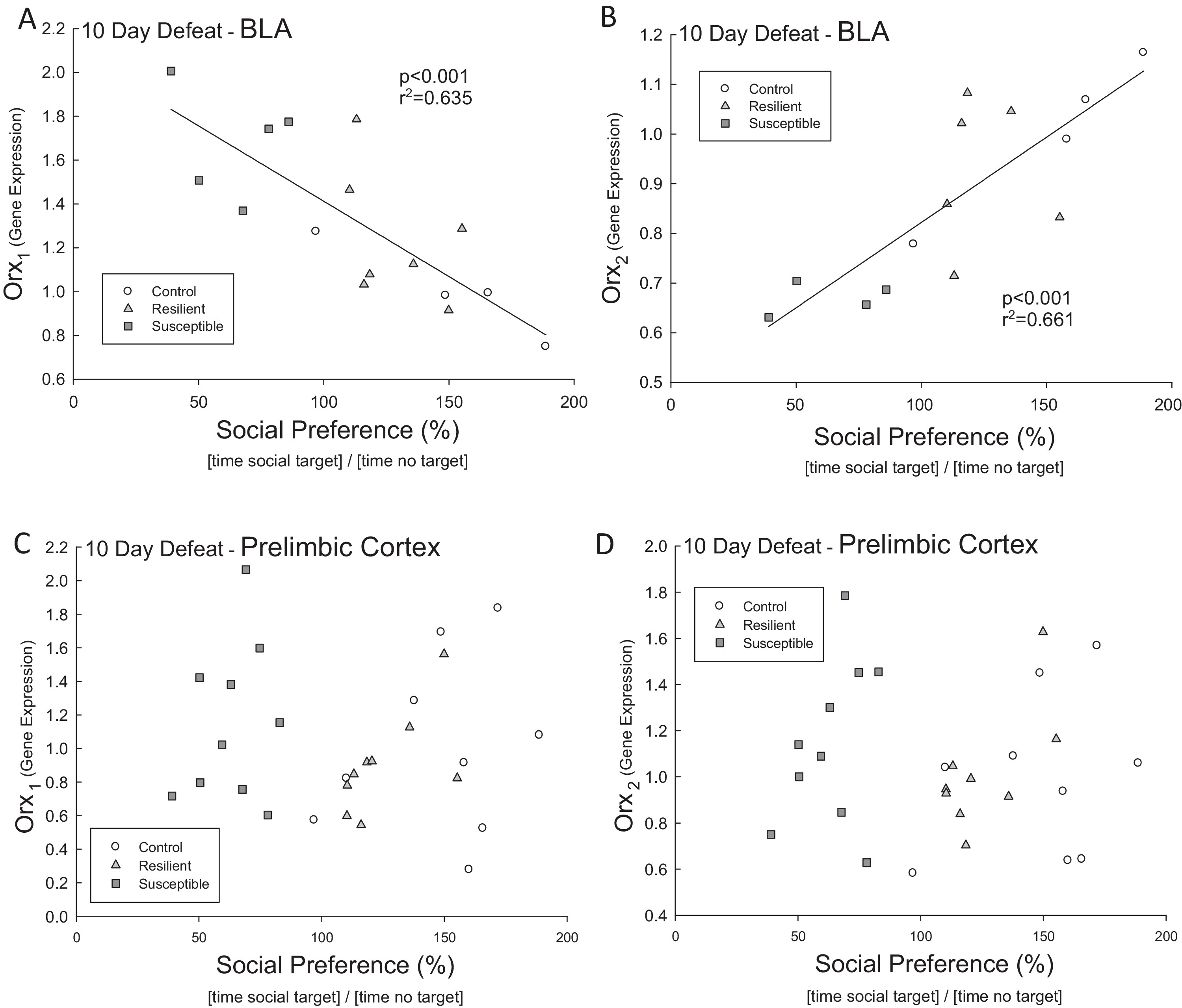 |
Figure 3. Orx1 negatively correlated with resilience Orx2 positively correlated Social preference scores were significantly correlated with BLA expression of (A) Orx1 and (B) Orx2 mRNA. In the BLA there was a significant (p < 0.001, r2 = 0.635) negative regression between the Orx1 mRNA and social preference, and a significant (p < 0.001, r2 = 0.661) positive regression between the Orx2 mRNA and social preference. These correlations were absent in the PrL for (C) Orx1 and (D) Orx2 receptor transcripts. |
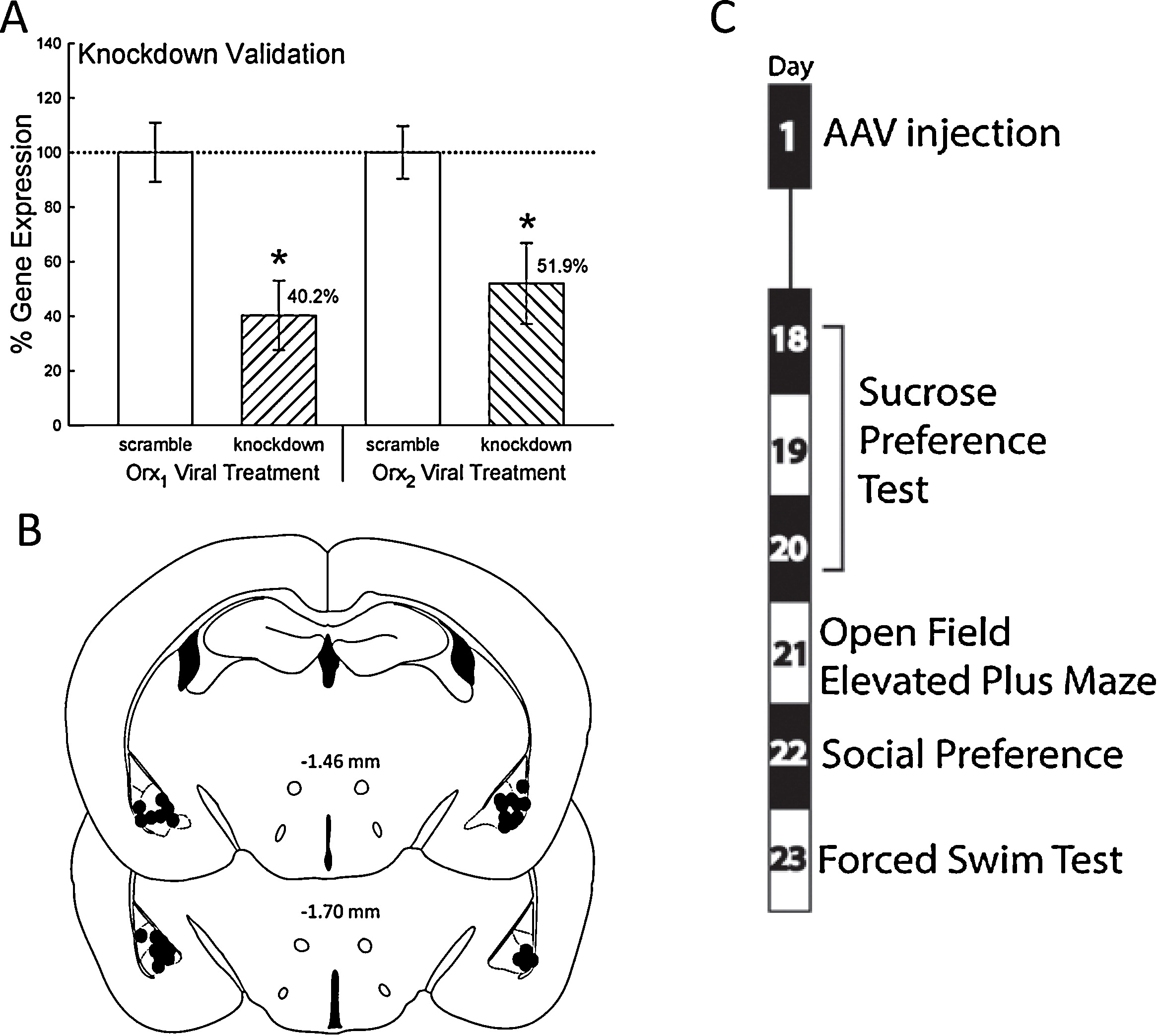 |
Figure 4.BLA IC viral injections of shRNA Viral shRNA constructs significantly (*) reduced the expression of the endogenous mRNA sequence for the respective orexin receptor. (A) Gene expression comparisons were made between the control group that received a nonspecific “scramble” virus and the experimental group which received the “knockdown” virus targeting the Orx1 or Orx2 receptor. (B) Behavioral data were analyzed for animals with expression of GFP (?) in the basolateral complex. (C) Sequence and timing of a series of tests commonly used to detect depression and anxiety related behaviors to which transfected animals were exposed. *p < 0.05. |
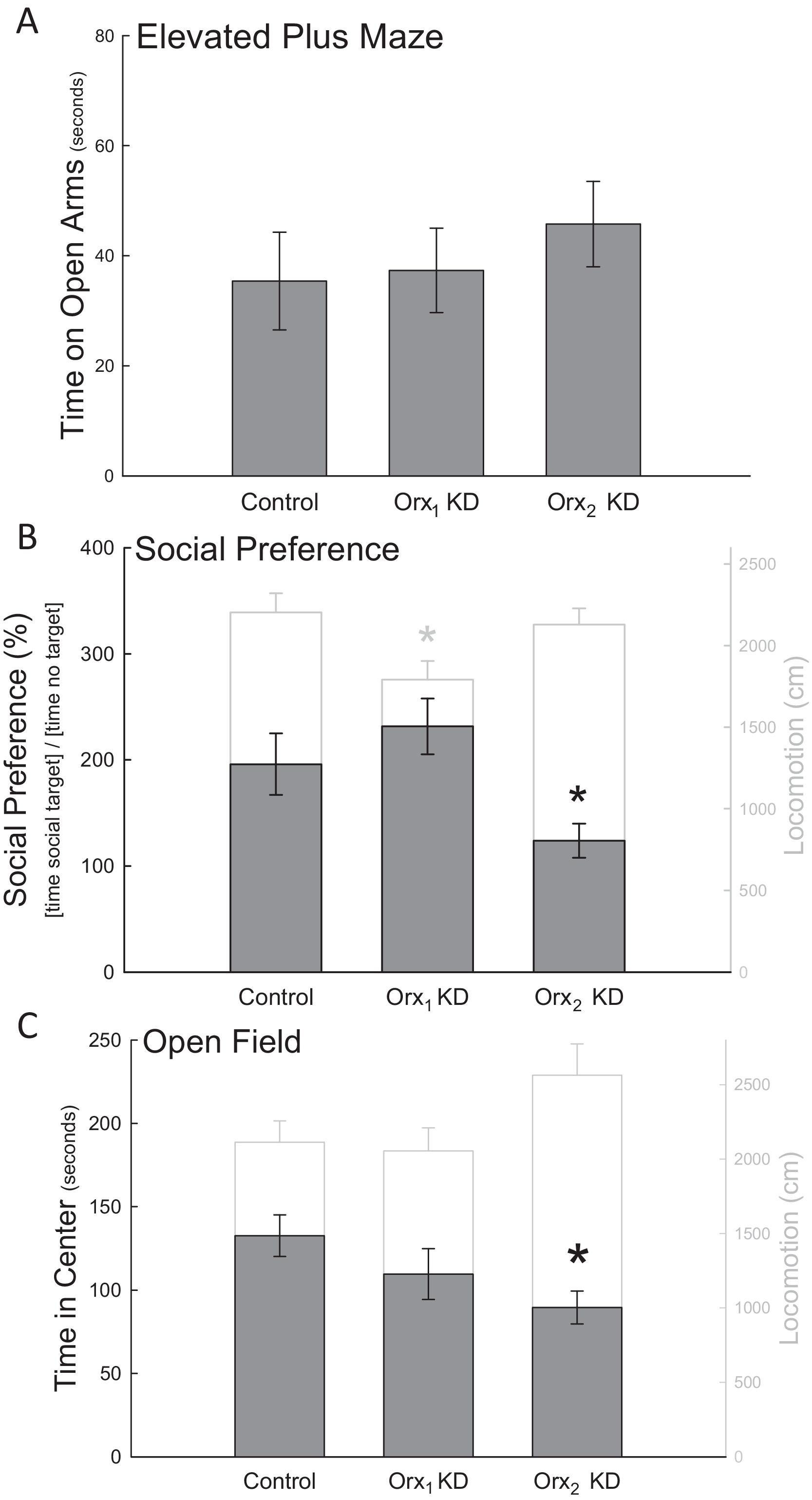 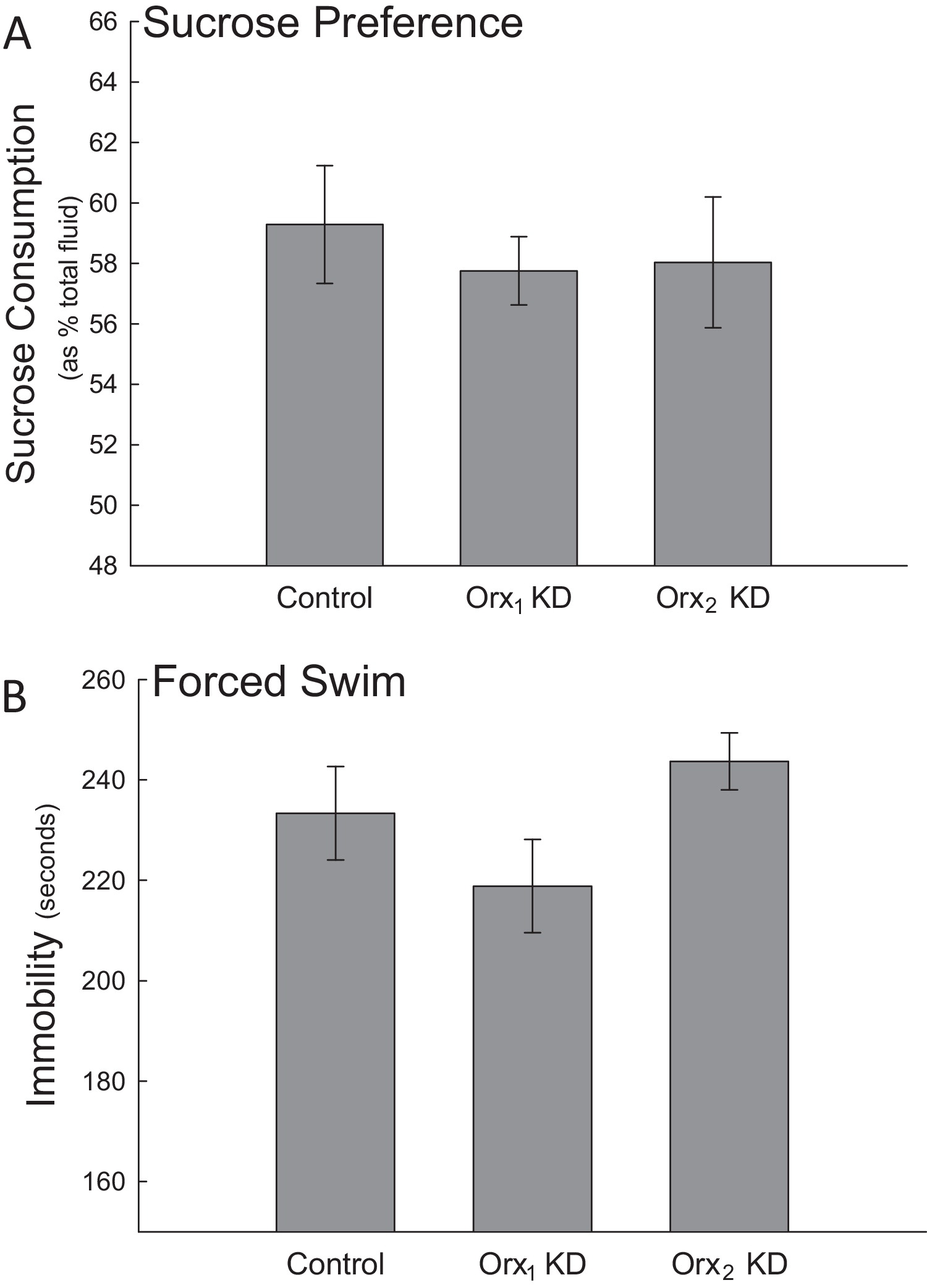 |
Figures 5 & 6. Orexin 2 receptors in the BLA are anxiolytic Knocking down the Orx2 receptor in the BLA increased anxiety-like behaviors. (A) While none of the treatment groups exhibited altered EPM performance, (B) the viral shRNA Orx2 knockdown significantly decreased (*) social preference relative to controls. Locomotion was unaffected in the social preference test with Orx2 knockdown. There was no significant change in social preference for animals that received the Orx1 knockdown despite a significant decrease in the total amount of locomotor activity. (C) Orx2 knockdown also decreased time spent in the center of an open field apparatus. There were no significant changes in locomotion due to Orx1 or Orx2 knockdown in the open field. *p < 0.05. Knockdown of either orexin receptor in the BLA produced no significant differences in behavioral measures of depression for either the (A) sucrose preference test or (B) forced swim test relative to the control treatment. |
 NIH R15 MH104485 Amygdalar Orexin Modulation of Affective Disorders $426,477 2015-2017
NIH R15 MH104485 Amygdalar Orexin Modulation of Affective Disorders $426,477 2015-2017
Published papers resulting from NIH R15 MH125306
Carpenter RE, B Sabirzhanov, TR Summers, TG Clark, J Keifer and CH Summers 2023 Anxiolytic reversal of classically conditioned /
chronic stress-induced gene expression and learning in the Stress Alternatives Model. Behavioural Brain Research
114258: 440: 1-10
Yaeger JDW, KT Krupp, BM Jacobs, BO Onserio, BL Meyerink, JT Cain, PJ Ronan, KJ Renner, RJ DiLeone, CH Summers 2022
Orexin 1 receptor antagonism in basolateral amygdala shifts the balance from pro- to anti-stress signaling and behavior.
Biological Psychiatry 91: 841-852
Yaeger JDW, KT Krupp, TR Summers, CH Summers 2022 Contextual generalization of social stress learning is modulated by
orexin receptors in basolateral amygdala. Neuropharmacology 215: 109168:1-12
Arendt DH, PJ Ronan, KD Oliver, LB Callahan,TR Summers and CH Summers 2013 Depressive
behavior and activation of the orexin/hypocretin system Behav Neurosci 127:86-94
Arendt DH, J Hassell, Hao Li, JK Achua, DJ Guarnieri, RJ DiLeone, PJ Ronan, and CH Summers 2014
Anxiolytic function of the orexin 2 /hypocretin A receptor in the basolateral amygdala.
Psychoneuroendocrinology 40C: 17-26
Keifer J and CH Summers 2016 Putting the "biology" back into "neurobiology: The strength of
diversity in animal model systems for neuroscience research Front Syst Neurosci 10: 69: 1-9
Korzan WJ and CH Summers 2021 Evolution of stress responses refine mechanisms of social rank.
Neurobiology of Stress 100328: 1-19
Pang T, JDW Yaeger, CH Summers, and R Mitra 2021 Cardinal role of the environment in stress induced changes
across life stages and generations. Neuroscience and Biobehavioral Reviews 124: 137-150
Pearson BL, JN Crawley, D Eilam, NS Pentkowski, CH Summers 2017 Curiosity as an approach to
ethoexperimental analysis: Behavioral neuroscience as seen by students and colleagues of Bob Blanchard.
Neuroscience & Biobehavioral Reviews 76: 415-422
Smith JP, MA Prince, JK Achua, JM Robertson, RT Anderson, PJ Ronan, CH Summers 2016 Intensity of anxiety is modified via complex
integrative stress circuitries. Psychoneuroendocrinology 63: 351-361
Robertson JM, JK Achua, JP Smith, MA Prince, CD Staton, PJ Ronan, TR Summers, CH Summers 2017 Anxious behavior induces
elevated hippocampal Cb2 receptor gene expression. Neuroscience 352: 273-284
Robertson JM, MA Prince, JK Achua, RE Carpenter, DH Arendt, JP Smith, TL Summers, TR Summers, CH Summers 2015 Nuance and behavioral
cogency: How the Visible Burrow System inspired the Stress-Alternatives Model and conceptualization of the continuum of anxiety.
Physiology and Behavior 146: 86-97
Staton CD, JDW Yaeger, DD Khalid, F Haroun, BS Fernandez, JS Fernandez, BK Summers, TR Summers, M Sathyanesan,
SS Newton, CH Summers 2018 Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes
susceptibility, to social stress, anxiety and depression. Neuropharmacology 143: 79-94.
Summers CH, JDW Yaeger, CD Staton, DH Arendt, TR Summers 2020 Orexin/hypocretin receptor modulation of anxiolytic and antidepressive
responses during social stress and decision-making: potential for therapy. Brain Research 1731: 146085: 1-15
Summers TR, TL Summers, RE Carpenter, JP Smith, SL Young, B Meyerink, TZ Orr, DH Arendt, and CH Summers 2017 Learning and CRF-induced
indecision during escape and submission in rainbow trout during socially aggressive interactions in the Stress-Alternatives Model
Frontiers in Neuroscience 11: 515: 1-13
Waters RP, M Rivalan, D Bangasser, J Deussing, M Ising, SK Wood, F Holsboer, CH Summers 2015 Evidence for the role of
corticotropin-releasing factor in major depression disorder Neuroscience & Biobehavioral Reviews 58: 63-78
Yaeger JDW, KT Krupp, JJ Gale, CH Summers 2020 Counter-balanced microcircuits for Orx1 and Orx2 regulation
of stress reactivity. Medicine in Drug Discovery 100059: 1-20
| Research Web Pages: Orexin, Anxiety, and Depression Stress Alternatives Model Anxiety Intensity Gradient |
| 3D-SAM Rainbow Trout Evolution of Anxiety and Depression Gene Sequences |
| Summers' Lab Home Investigators Publications Cliff Summers Contact Us |
|
Neuroscience Group at USD
Biology Department
Summers' Biology Webpage
Summers' Course Offerings
Contact Us
|